One of the most remarkable and novel discoveries in the last 400 years was electricity. We might ask, “Has electricity been around that long?” The answer is yes, and perhaps much longer. Its practical use has only been at our disposal since the mid to late 1800s, and in a limited way at first. Some of the earliest public works gaining attention were streets lights in Berlin in 1882, lighting up the Chicago World’s Fair in 1893 with 250,000 light bulbs, and illuminating a bridge over the river Seine during the Paris 1900 World Fair.
The use of electricity may go back further. While constructing a railway in 1936 near Baghdad, workers uncovered what appeared to be a prehistoric battery, also known as the Parthian Battery. The object dates back to the Parthian empire and is believed to be 2,000 years old. The battery consisted of a clay jar that was filled with a vinegar solution into which an iron rod surrounded by a copper cylinder was inserted. This device produced 1.1 to 2.0 volts of electricity. Figure 1 illustrates the Parthian Battery.
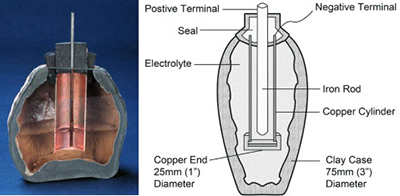
Figure 1: Parthian Battery.
A clay jar of a prehistoric battery holds an iron rod surrounded by a copper cylinder.
When filled with vinegar or electrolytic solution, the jar produces 1.1 to 2 volts.
Not all scientists accept the Parthian Battery as a source of energy. It is possible that the device was used for electroplating, adding a layer of gold or other precious metals to a surface. The Egyptians are said to have electroplated antimony onto copper over 4,300 years ago. Archeological evidence suggests the Babylonians were the first to discover and employ a galvanic technique in the manufacturing of jewelry by using an electrolyte based on grape juice to gold-plate stoneware. The Parthians, who ruled Baghdad (ca. 250 BC), may have used batteries to electroplate silver.
One of the earliest methods to generate electricity in modern times was by creating a static charge. In 1660, Otto von Guericke constructed an electrical machine using a large sulfur globe which, when rubbed and turned, attracted feathers and small pieces of paper. Guericke was able to prove that the sparks generated were electrical in nature.
In 1744, Ewald Georg von Kleist developed the Leyden jar that stored static charge in a glass jar that was lined with metallic foil on the inside and outside of the container. Many scientists, including Peter van Musschenbroek, professor at Leiden, the Netherlands, thought that electricity resembled a fluid that could be captured in a bottle. They did not know that the two metallic foils formed a capacitor. When charged up with high voltage, the Leyden jar gave the gentlemen an unexplainable hefty shock when they touched the metallic foil.
The first practical use of static electricity was the “electric pistol” that Alessandro Volta (1745–1827) invented. He thought of providing long-distance communications, albeit only one Boolean bit. An iron wire supported by wooden poles was to be strung from Como to Milan, Italy. At the receiving end, the wire would terminate in a jar filled with methane gas. To signal a coded event, an electrical spark would be sent by wire to detonate the jar. This communications link was never built. Figure 2 shows a pencil rendering of Alessandro Volta.

Figure 2: Alessandro Volta, inventor of the electric battery.
Volta’s discovery of the decomposition of water by an electrical current laid the foundation of electrochemistry.
In 1791, while working at Bologna University, Luigi Galvani discovered that the muscle of a frog would contract when touched by a metallic object. This phenomenon became known as animal electricity. Prompted by these experiments, Volta initiated a series of experiments using zinc, lead, tin and iron as positive plates (cathode); and copper, silver, gold and graphite as negative plates (anode). The interest in galvanic electricity soon became widespread.
Early Batteries
Volta discovered in 1800 that certain fluids would generate a continuous flow of electrical power when used as a conductor. This discovery led to the invention of the first voltaic cell, more commonly known as battery. Volta learned further that the voltage would increase when voltaic cells were stacked on top of each other. Figure 3.1 and 3.2 illustrate such a series connection.
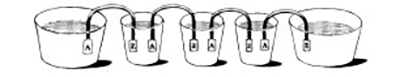
Figure 3.1: Volta’s experiments with the electric battery in 1796.
Silver (A) and zinc (Z) metals are immersed in cups filled with electrolyte and connected in series.
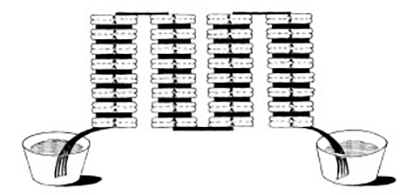
Figure 3.2: Volta’s experiments with the electric battery in 1796.
Silver and zinc electrodes are connected in series, separated by paper soaked with electrolyte.
Volta’s experiments with the electric battery in 1796.
Metals in a battery have different electron affinities. Volta noticed that the voltage potential of dissimilar metals became stronger the farther apart the affinity numbers moved. The first number in the metals listed below demonstrates the affinity to attract electrons; the second is the oxidation state.
- Zinc = 1.6 / -0.76 V
- Lead = 1.9 / -0.13 V
- Tin = 1.8 / -1.07 V
- Iron = 1.8 / -0.04 V
- Copper = 1.9 / 0.159 V
- Silver = 1.9 / 1.98 V
- Gold = 2.4 / 1.83 V
- Carbon = 2.5 / 0.13 V
The metals determine the battery voltage; they were separated with moist paper soaked in salt water.
In the same year, Volta released his discovery of a continuous source of electricity to the Royal Society of London. No longer were experiments limited to a brief display of sparks that lasted a fraction of a second; an endless stream of electric current now seemed possible.
France was one of the first nations to officially recognize Volta’s discoveries. This was during a time when France was approaching the height of scientific advancements. New ideas were welcomed with open arms as they helped to support of the country’s political agenda. In a series of lectures, Volta addressed the Institute of France. Napoleon Bonaparte participated in the experiments, drawing sparks from the battery, melting a steel wire, discharging an electric pistol and decomposing water into its elements (see Figure 4).

Figure 4: Volta’s experimentations at the Institute of France.
Volta’s discoveries so impressed the world that in November 1800 the Institute of France invited him to lecture at events in which Napoleon Bonaparte participated. Napoleon helped with the experiments, drawing sparks from the battery, melting a steel wire, discharging an electric pistol and decomposing water into its elements.
In 1800, Sir Humphry Davy, inventor of the miner’s safety lamp, began testing the chemical effects of electricity and found out that decomposition occurred when passing electrical current through substances. This process was later called electrolysis.
He made new discoveries by installing the world’s largest and most powerful electric battery in the vaults of the Royal Institution of London, connecting the battery to charcoal electrodes produced the first electric light. Witnesses reported that his voltaic arc lamp produced “the most brilliant ascending arch of light ever seen.”
In 1802, William Cruickshank designed the first electric battery for mass production. He arranged square sheets of copper with equal-sized sheets of zinc placed into a long rectangular wooden box and soldered together. Grooves in the box held the metal plates in position. The sealed box was then filled with an electrolyte of brine, or watered-down acid. This resembled the flooded battery that is still with us today. Figure 5 illustrates his battery workshop.

Figure 5: Cruickshank and the first flooded battery.
William Cruickshank, an English chemist, built a battery of electric cells by joining zinc and copper plates in a wooden box filled with an electrolyte solution. This flooded design had the advantage of not drying out with use and provided more energy than Volta’s disc arrangement.
Invention of the Rechargeable Battery
In 1836, John F. Daniell, an English chemist, developed an improved battery that produced a steadier current than earlier attempts to store electrical energy. In 1859, the French physician Gaston Planté invented the first rechargeable battery based on lead acid, a system that is still used today. Until then, all batteries were primary, meaning they could not be recharged.
In 1899, Waldmar Jungner from Sweden invented the nickel-cadmium (NiCd) battery that used nickel as the positive electrode (cathode) and cadmium as the negative (anode). High material costs compared to lead limited its use. Two years later, Thomas Edison replaced cadmium with iron, and this battery was called nickel-iron (NiFe). Low specific energy, poor performance at low temperature and high self-discharge limited the success of the nickel-iron battery. It was not until 1932 that Schlecht and Ackermann achieved higher load currents and improved the longevity of NiCd by inventing the sintered pole plate. In 1947, Georg Neumann succeeded in sealing the cell.
For many years, NiCd was the only rechargeable battery for portable applications. In the 1990s, environmentalists in Europe became concerned about the harm incurred when NiCd is carelessly disposed. The Battery Directive 2006/66/EC now restricts the sale of NiCd batteries in the European Union except for specialty industrial use for which no replacement is suitable. The alternative is nickel-metal-hydride (NiMH), a more environmentally friendly battery that is similar to NiCd.
Most research activities today revolve around improving lithium-based systems, first commercialized by Sony in 1991. Besides powering cellular phones, laptops, digital cameras, power tools and medical devices, Li-ion is also used for electric vehicles and satellites. The battery has a number of benefits, most notably its high specific energy, simple charging, low maintenance and being environmentally benign.
Electricity Through Magnetism
Generating electricity through magnetism came relatively late. In 1820, André-Marie Ampère (1775–1836) noticed that wires carrying an electric current were at times attracted to, and at other times repelled from, one another. In 1831, Michael Faraday (1791–1867) demonstrated how a copper disc provided a constant flow of electricity while revolving in a strong magnetic field. Faraday, assisting Humphry Davy and his research team, succeeded in generating an endless electrical force as long as the movement between a coil and magnet continued. This led to the invention of the electric generator, as well as the electric motor by reversing the process.
Shortly thereafter, transformers were developed that converted alternating current (AC) to any desired voltage. In 1833, Faraday established the foundation of electromagnetism on which Faraday’s law is based. It relates to electromagnetism found in transformers, inductors and many types of electrical motors and generators. Once the relationship with magnetism was understood, large generators were built to produce a steady flow of electricity. Motors followed that enabled mechanical movement and Thomas Edison’s light bulb appeared to conquer darkness.
Early electrical plants produced direct current (DC) with distribution limitations of 3km (~2 miles) from the plant. In around 1886, the Niagara Falls Power Company (NFPC) offered $100,000 for a method to transmit electricity over a long distance. After much controversy and failed proposals, the world’s brightest minds met in London, England, and the prize was awarded to Nikola Tesla (1856–1943), a Serbian immigrant who created the AC transmission system. NRPC with Tesla as a consultant built a multi-phase AC system, delivering power from new Niagara power station as far as Buffalo, NY.

Figure 6: Nikola Tesla (1856–1943).
Serbian-American physicist, inventor and engineer best known for alternating current supply systems and rotating magnetic fields.
DC systems run on low voltage and require heavy wires; AC could be transformed to higher voltages for transmission over light wires and then reduced for use. Older folks supported DC while younger geniuses gravitated towards AC. Thomas Edison was dead set against AC, giving danger by electrocution as a reason.
The disagreement continued, but AC became the accepted norm that was also supported by Europe. George Westinghouse, an American inventor and manufacturer, began developing the Tesla system to the displeasure of Thomas Edison.
To everyone’s amazement, AC power lit up the Chicago World Fair in 1893 (Figure 7). Westinghouse then built three large generators to transform energy from the Niagara Falls to electricity. Three-phase AC technology developed by Tesla enabled the transmission of electric power over great distances cheaply. Electricity was thus made widely available to humanity to improve the quality of life.

Figure 7: 250,000 light bulbs illuminate the Chicago World Fair in 1893, also known as Chicago’s World Columbian Exposition.[1]
The success of the electric light led to building three large hydro generators at Niagara Falls.
Telecommunications by wire that was strung along railways operated mostly by primary batteries that needed frequent replacement. Telex, an early means to transmit data, was digital in that the batteries activated a series of relays. The price to send a message was based on the number of relay clicks required.
In the mid-1800s, telegraphy opened new careers for bright young men. Staff operating these devices moved into the growing middle class, far removed from mills and mines burdened with labor, dirt and danger. Steel magnate Andrew Carnegie recalled his early days as a telegraphy messenger: Alfred Hitchcock started his career as an estimator before becoming an illustrator.
The invention of the electronic vacuum tube in the early 1900s formed the significant next step towards high technology. It enabled frequency oscillators, signal amplifications and digital switching. This led to radio broadcasting in the 1920s and the first digital computer, called ENIAC, in 1946. The invention of the transistor in 1947 paved the way for the arrival of the integrated circuit 10 years later, and the microprocessor that ushered in the Information Age. This forever changed the way we live and work.
Humanity has become dependent on electricity and with increased mobility, people gravitate towards portable power involving the battery. As the battery improves further, more tasks will be made possible with this portable power source.

What Would Happen If We Didn’t Invent Batteries?
If the battery didn’t exist, we’d have no way to store electricity. If we couldn’t store electricity, our society would be drastically different than it is now. Without energy storage, we would be completely reliant on the energy grid. Every device we own would still require an outlet in order to run. Cell phones wouldn’t exist, cars wouldn’t run as we know them, and we’d have no renewable energy. Yes, we would have electricity, but it wouldn’t be mobile.
Fortunately, scientists invented and developed the battery, and continue to improve and upgrade them today. Basen Batteries is proud to be part of the advancement of this technology. Join us on Facebook, Twitter, and YouTube to learn more about how LiFePO4 battery systems can power your life.